Oxygen has electronic configuration [He]2s22p4, and has common oxidation states -2,-1, and 0. Other members of the group show a tendency for hypervalence, and have oxidation states from +1 to +6, eg. sulphur in SF6. (For a comparison of the chemistries of oxygen and sulphur, click here.)
There are two common allotropes of Oxygen: O2 and O3 (Ozone).
O2
In its electronic ground state, this is paramagnetic (has unpaired electrons), has electron spin S=1 (it has a triplet ground state), and forms a pale blue liquid and solid at low temperatures
|
Ground State of O2: the 2πg antibonding orbitals are each singly occupied, giving an electron spin of 1. This is therefore a triplet state, with term symbol 3Σg–. |
|
| The excited state of O2 has the electrons in the 2πg antibonding orbitals paired, leading to the singlet state, 1Δg. |
The paired electrons can take part in concerted Diels-Alder type reactions: |
O2 can be reduced to give O2+, and oxidized to give O2– and O22-.
In the case of O2+, the electron is removed from an antibonding orbital, and in the other cases the extra electrons are added to antibonding orbitals. The effect this has on the bond order, and therefore bond strength, is shown in the table.
| Species | No. of bonding electrons (NB) | No. of antibonding electrons (NA) | Bond Order: (NB-NA/2) |
| O2+ | 6 | 1 | 2.5 |
| O2 | 6 | 2 | 2 |
| O2– | 6 | 3 | 1.5 |
| O22- | 6 | 4 | 1 |
O2 is a π-acid like N2 and CO, and so can act as a ligand in the same way. This is very important as the transmission of oxygen in blood is by the reversible binding of molecular O2 (as O2–) to Fe atoms in haemoglobin. O2 also binds to other transition metals, eg. in the formation of Vaska’s complex:
| Formation of Vaska’s complex |
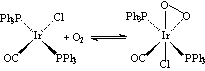 |
O3: Ozone
This is prepared by electrical discharge through O2, and has the bent structure predicted by VSEPR theory with two resonance forms. It is highly reactive, with a high positive Gibbs free energy of formation.
| Resonance structure of Ozone: |
Stereochemistry of Oxygen
Unicoordinate O: E=O bonds are very common
Bicoordinate O: E-O-E groups are typically bent (as predicted by VSEPR), but the formation of Edπ-Opπ interactions tend to favour linear arrangements.
Tricoordinate O: this is typically pyramidal, eg. in +OR3, but is trigonal planar in some OM3 complexes.