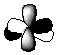We now know, from our previous discussion, that the electrons orbit the nucleus at a very large distance (on a nuclear scale), but what is the nature of the “path” they follow?
An electron orbiting a nucleus is described by a wavefunction.
This is contrary to the common image of electrons being like little balls orbiting the nucleus, for quantum mechanics tells us that there is no precise location of the electron.
The wavefunction tells us where the electron is likely to be found (more precisely, the square of the wavefunction is proportional to the probability of finding the electron at that point).
One of the most important discoveries of modern chemistry was the realisation that electrons are not completely free to choose their path, but must stay in certain regions according to defined energy levels. The electrons may be thought of as orbiting the nucleus in shells. Within each shell electrons are grouped into subshells, and within each shell, electrons are paired up into orbitals: In the diagram below, the electrons are represented by single-headed arrows, and the orbitals are denoted by horizontal lines.
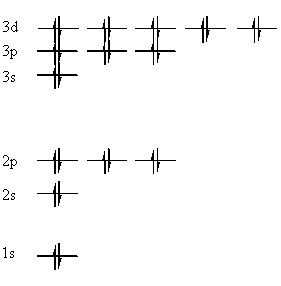 |
The quantum mechanics behind the reasons the electrons adopt this pattern of orbitals, subshells and shells is discussed in the physical chemistry section. The shells, as we can see from the diagram above, are denoted 1,2,3 etc in order of increasing energy. The subshells are denoted s,p,d,f for historical reasons. An s subshell has 1 orbital, a p subshell has 3, a d subshell has 5, and an f subshell has 7. |
Let us now look at some depictions of orbitals. When representing an orbital, it is important to remember that it is impossible to accurately “draw” an orbital.
The representations below are defining boundaries that contain the volume in which the electron is most likely to be found.
An s orbital is simply a sphere:
A p orbital is a dumbell shape:
The three p orbitals together are all orthogonal to each other. They are designated x,y and z:
Most of organic chemistry is only concerned with s and p orbitals, as the elements most commonly encountered in organic chemistry do not have enough electrons to fill anything beyond the 2p subshell. One facet of the shape of a p orbital is that it has a nodal plane through it’s centre, i.e. in the centre of the nucleus. In this region, there is no chance of finding the electron. s orbitals do not have any nodal planes