We now know what the orbitals of a general atom look like, but now we need to know in what order the orbitals are filled on progressing through the periodic table, so we can then make predictions about those elements.
The following rules can be used to predict the lowest energy state of the electronic configuration (the ground state):
1. Orbitals of lowest energy are filled first. The order of orbital energy is: 1s, 2s, 2p, 3s, 3p, 4s, 3d… etc.
 |
The diagram above can be used as an aid to remembering the order in which the orbitals are filled. Start with the top arrow, and then following the direction of the arrow, scan down successive arrows, making a note of the subshells passed in order.
2. A maximum of two electrons can occupy each orbital, and they must have opposite spins to do so. This is a consequence of the Pauli principle. Schematically, this is represented by drawing single-headed arrows pointing in opposite directions. e.g.:
| Allowed | Forbidden |
3. If two or more orbitals have the same energy (i.e. are degenerate), and are empty, all the degenerate orbitals will fill with one electron before any of them fills with two.
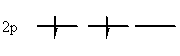 |
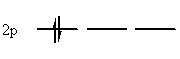 |
| Allowed | Not allowed |
Let us now use these rules to predict the ground state of carbon:
For carbon, we know that Z=6, so there must be 6 electrons orbiting the nucleus. Using the first rule, we know that the 1s orbital will fill first, followed by the 2s. Between them, they take 4 electrons. There is no degeneracy to worry about yet, so we do not yet need the third rule. The remaining two electrons must therefore go in the next lowest energy subshell, which is the 2p. Now we use the third rule, to predict that the electrons will have parallel spins, and will be in separate orbitals of the 2p subshell:
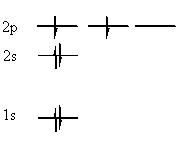 |